Maintaining biodiversity and ecosystem services through metasystems thinking
Empirical examples of metasystems dynamics
Chapter 3 of my unpublished book, Maintaining biodiversity and ecosystem services through metasystems thinking in a changing world, can be read below. Here at Substack, excerpts of the book will be published, but abridged and adapted to attract an audience broader than ecologists only.
Empirical examples of metasystems dynamics
3.1. General overview
Sets of many different ecosystems may act as metasystems, but it is hard to find examples better than freshwater ecosystems embedded within a drainage basin with which to illustrate metacommunity and metaecosystem dynamics. The characteristics of drainage basins, smaller catchments and individual freshwater ecosystems, however, present both challenges and opportunities for studying metasystems. Challenges include making representative observations in spatially heterogeneous and temporally highly dynamic systems. Opportunities include studying more or less spatially distinct and isolated ecosystems scattered in the landscape or, figuratively speaking, ‘aquatic islands in the sea of terrestrial environments’.
Two major types of freshwater ecosystems, including lotic (rivers and streams) and lentic (lakes and ponds) are typically considered separately because the presence or absence of unidirectional water flow is a major hydro-ecological factor that distinguishes between lotic and lentic ecosystems (Heino and others 2015a). This division is admittedly very general, as there are highly different rivers worldwide, depending on the geographical location of the drainage basin, as well as the geomorphological and climate conditions where the drainage basin is located (for a recent comparison, see Snåre and others 2024). Similarly, lakes in boreal areas and in the tropics are, for instance, highly different ecosystems when it comes to physical, chemical and biological dynamics. These features should be emphasized because they provide the template upon which metasystem dynamics can be seen and how they vary in space and time. Indeed, context dependence, or the understanding that many, if not most, ecological phenomena vary between areas, ecosystems and organisms studied (Heino and others 2012), is a common finding in ecological research on metasystems. Ironically, as emphasized below and in Chapter 2, context dependence may be the most ‘general law’ in ecology, a field of science that is generally searching for generalities.
In the following abridged account, the focus is on riverine metasystems only.
Figure 3-1. A boreal river showing the spatial variation in environmental conditions even along a short reach of the river. Photo taken by the author in northern Finland.
3.2. Metasystems of riverine networks
Riverine ecosystems within a drainage basin are prime examples of natural networks, where smaller streams join with consecutively larger streams and rivers, eventually entering the sea through a large mainstem river (Tonkin and others 2018). In riverine networks, the movements of organisms and matter are omnipresent and vary considerably depending on the location in the network that is under investigation. For example, the River Continuum Concept (RCC) predicts changes in the importance of allochthonous (i.e., matter produced outside of the focal ecosystem) and autochthonous (i.e., matter produced within the focal ecosystem) production for ecosystem structure and function, and for biotic communities, from the upstream to downstream direction (Vannote and others 1980). According to the RCC, allochthonous leaf material shed from riparian trees contributes disproportionately to organic matter budgets of small headwater streams in forested areas (Figure 3-2, Video 3-1). In contrast, the importance of allochthonous production diminished in mid-sized rivers that are assumed to be dominated by autochthonous algal productivity. In large sluggish-flowing lowland rivers, allochthonous inputs may again gain increasing importance but mainly as fine particulate organic matter produced in upstream areas. Simultaneously to changes in allochthonous versus autochthonous production, also the organisms adapted to utilize different resources vary longitudinally (Cummins and Klug 1979). Chief among the organisms that have been studied extensively are aquatic macroinvertebrates (Wallace and Webster 1996) and fish (Matthews 1998). As this topic is widely covered in textbooks on stream ecology (Allan and others 2021), it will not be considered more widely herein.
Figure 3-2. Headwater streams, such as the one depicted above, are intimately associated with their riparian zones, which are providing organic material as leaf litter entering the stream ecosystem. Aquatic insects emerging from the stream are, in turn, proving prey for terrestrial insectivores such as many species of birds, bats, lizards and predaceous invertebrates in the riparia (Soininen and others 2015). Riparian corridors are used as dispersal routes by many aquatic and terrestrial insects, as well as birds and mammals among many other organisms (Ament and others 2014). Note that the water of the exemplary stream is not polluted, but the brown water colour mainly results from humic substances originating in the catchment with boreal peatlands. Photo was taken by the author in northern Finland.
Video 3-1. This video was recorded at a location downstream of that in the photo above. While the photo was taken in early August, the video was recorded in late September. The video therefore exemplifies the autumnal leaf fall entering the stream and shows the influences of wind speed and current velocity on the deposition of the leaves on the margins of the stream. Listen to the voices of the birds that were actively foraging close to the stream, but not as much in the nearby upland forests. Video recorded by the author in northern Finland.
Riverine networks are even more complex than the mere longitudinal upstream-downstream water flow might suggest. Different processes and abiotic-biotic interactions also occur laterally, vertically and temporally (Ward 1998). Laterally, sidearms of rivers and seasonally flooded areas close to the river may act as important spawning and nursery sites for fish, whereas many invertebrate organisms may benefit from shallow and warm areas with plentiful organic material in flooded areas. Vertically, some organisms may occur deep in the sediments, others just below the sediment surface, some dwell on stones or within vegetation, while others swim freely in the water column. Temporally, rivers in subtropical are known to exhibit distinct periods of ‘dry’ and ‘wet’ seasons, with consequent effects on population, community and ecosystem dynamics, eventually shaping the dynamics of entire metasystems (Li and others 2020). In temperate, boreal and Arctic areas, flooding following autumnal rains and/or after the snowmelt in the spring have distinct effects of the ecological and hydrological dynamics of rivers and streams. On a larger temporal window, year-to-year variation in temperature and precipitation shapes riverine ecosystem features, with some years being notorious for being extremely dry, while others are plagued by problems with excess flooding even destroying infrastructure. Longitudinal, lateral, vertical and temporal dynamics affect biotic communities and environmental processes within river and stream ecosystems, eventually extending beyond to the aquatic ecosystems to riparian and marine ecosystems.
For biotic communities, riverine networks comprise a large number of headwater streams, which account for most of the overall network length. Small streams are thus disproportionately more common than larger downstream rivers at the level of the entire riverine network, thereby having a high relevance for metapopulation, metacommunity and metaecosystem dynamics (Altermatt 2013). Headwater streams are typically relatively isolated, whereas mid-sized rivers and large rivers are more connected to other large tributaries and the mainstem river itself. If this is true in ecological terms in general, the importance of dispersal changes with the location in the river network under investigation. Spatial processes may thus be more important in headwater streams, especially when it comes to dispersal limitation (i.e., lack of dispersing organisms to colonize a location), whereas mainstem rivers by being more connected to other streams and rivers are assumed to be more strongly influenced by high numbers of dispersing organisms. An underlying reason and its consequences pertain to the likelihood of high dispersal rates masking environmental filtering, seen as ‘mass effects’ in mainstem rivers (see Chapter 2).
A holistic metacommunity approach that considers the dendritic nature of stream network structure may indeed provide interesting insights and predictions for riverine research. It has been hypothesized that isolated headwater streams receive fewer migrants, and local factors should therefore be the main forces controlling the composition of local biotic communities (Brown and Swan 2010). However, as larger rivers should receive migrants from both upstream and downstream areas as well as from tributaries, high dispersal rates and consequent mass effects should thus be of importance as well. As always, context dependence in the relative roles of dispersal-related and niche-related influences on biotic communities in riverine ecosystems is a common finding.
Headwater streams have been common subjects in aquatic metacommunity research. As emphasised above, they receive migrants from downstream reaches, as shown for strictly aquatic organisms (such as fish), as well from other headwater streams, as seen in organisms with flying adults (such as aquatic insects) or with adult phases (such as semi-aquatic frogs, salamanders and mammals) able to cross inhospitable terrestrial environments using other means. A special example are first-order headwater streams located at the periphery of riverine networks that may be mostly colonized from downstream areas (Auerbach and Poff 2011), especially when it comes to obligatory aquatic organisms such as fish and shrimps. The high degree of isolation of headwaters in comparison to mainstem rivers is accentuated by the small population sizes of organisms, the latter being dictated by the small potential habitat area in a headwater stream. Accordingly, one could expect higher extinction rates, lower emigration rates, and more temporally variable species composition in headwater streams compared to consecutively larger rivers in the central parts of a drainage basin. Findings following this line of reasoning have been observed for stream fishes (Gotelli and Taylor 1999). Also, the theoretically limited chances of headwater streams to receive migrants from elsewhere in the drainage basin may first and foremost cause high degrees of temporal variation in species composition, which may also be portrayed as distinct spatial differences in species composition among headwater streams (Clarke and others 2008). Such spatial differences, if they result from stochastic extinction and colonization, may lead to biotic community composition that is not associated with any environmental factor.
The limited chances in headwater streams for recolonisation after local extinctions have occurred may have important consequences for metacommunity studies that aim to account for variation in community composition using environmental features and spatial factors. There are several reasons why accounting for community compositional variation has proven to be challenging, including 1) missing environmental variables, 2) not accounting for biotic interactions, and 3) inadequate data analytical methods to account for spatial and temporal aspects of community variation (Heino and others 2015b). For example, studies that are based on snapshot-sampling (i.e., samples taken in only one period in a study), may not be able to predict community compositional variation by using local environmental variables only. Such spatio-temporal variation that cannot be associated with any measured environmental variables may result in low amounts of variation explained in community composition, a finding that is highly typical in riverine metacommunity studies. In other words, and as already suggested above, stochastic factors (e.g., rarely occurring colonization and frequent extinction events) may lead to distinct community compositional differences among sites (Siqueira and others 2020), and such differences may be hard, if not impossible, to account for by any measurable predictor variable.
Different kinds of riverine organisms exhibit a wide range of dispersal strategies, modes, abilities, and rates (Heino and others 2017). Hence, the likelihood to leave their natal locations and reach other locations that are suitable for them varies considerably among and within a given group of riverine organisms. There are various strategies of dispersal among aquatic organisms. These strategies vary from aquatic passive dispersal with stream flow (e.g., drift of insect larvae and other invertebrates), passive aquatic dispersal via animal vectors (e.g., fish hosts of mussels), active aquatic dispersal within streams and rivers (e.g., fish), overland passive dispersal with prevailing wind directions (e.g., microalgae), and overland passive dispersal via animal vectors (e.g., microalgae).
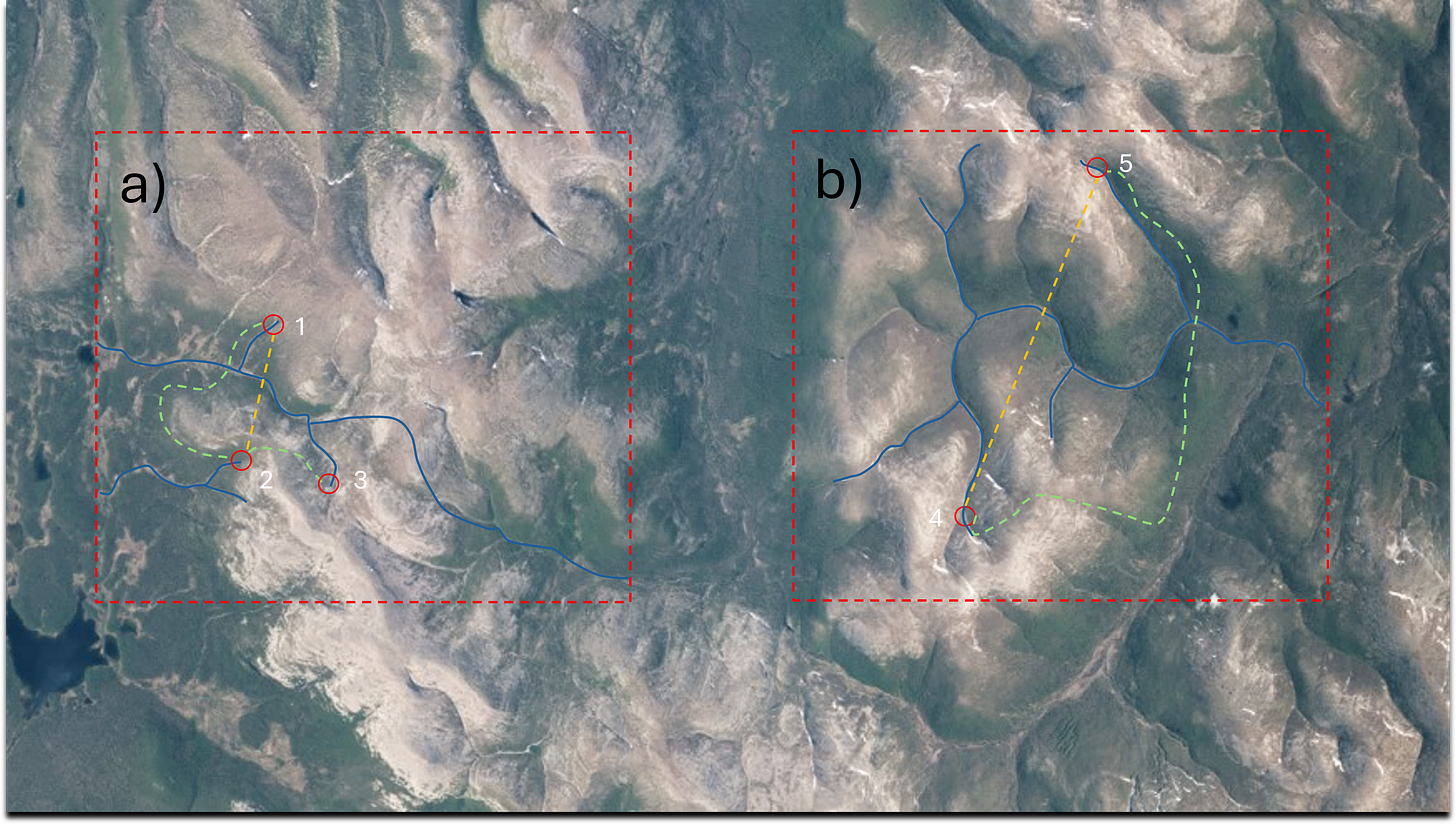
As a heuristic example, one can consider three hypothetical animal species, each having three different potential routes to disperse between sites (Figure 3-3). First, the fish (“as the fish swims”) can only move between sites via watercourses, unless deliberately transferred between sites during fish introductions or translocations. Second, the crow (“as the crow flies”) by being able to fly overland can choose the shortest path (i.e., shortest straight distance) between sites. Third, a wise mammal species, such as the fox (“as the fox runs”) can choose the least-cost path between sites to avoid spending too much energy when moving among locations (Kärnä and others 2015). The use of less costly paths is preferable but is only possible if the animal species in question can move easily on land and has previous information of the area (Ament and others 2014). To summarize, the optimal path or route to disperse between sites depends on various things, and all generalizations, especially when whole biotic communities are studied, are prone to lead to erroneous conclusions. This is because species within biotic communities, say aquatic macroinvertebrate communities, incorporate species that have widely different strategies and abilities of dispersal.
Figure 3-3. Examples of different hypothetical dispersal routes in a natural river catchment. On the left (a), the dispersal of obligatorily aquatic species, such as fish, occurs naturally only via the watercourse (marked in blue colour). In turn, species able to fly can use the shortest path between sites (marked in yellow colour). Another possible dispersal route can be used by animal species that are able to actively select the least-cost path (least-cost distance between locations 1 and 2 or between locations 2 and 3, marked in green colour). In practice, riparia and lowlands close to a river could be expected to be more suitable (i.e., less costly) routes compared with crossing upland areas (i.e., more costly) and going uphill and downhill. On the right (b), there are also three options. A dispersing organism could use watercourses (marked in blue colour), shortest overland path (marked in yellow colour), or least-cost path (marked in green colour) to reach site 5 from site 4. Depending on the dispersal strategies and abilities of the animal species in question, the optimal route varies regarding the landscape characteristics of the drainage basin.
Aquatic insects are among the most typical inhabitants of streams and rivers. They are capable of overland dispersal, yet their dispersal modes, abilities and distances vary considerably among species, which are notoriously difficult to estimate in field conditions. Some broad generalizations can be made, though. Many dragonflies are considered stronger fliers being able to fly longer distances compared with species in most other aquatic insect groups. In contrast, stoneflies, some of which have rudimentary or not-fully developed wings, may exhibit the other extreme end of ‘the dispersal ability gradient’ by being poor dispersers. However, our understanding of aquatic insect dispersal is highly underdeveloped (Bilton and others 2001), and most studies have been based on various proxies for dispersal rather than direct measures of dispersal rates or dispersal distances. These proxies are biological features of organisms, such as body size and wing muscle development, and geo-environmental factors, such as river forms and landscape surface features (Heino and others 2017).
The dispersal distances of riverine organisms other than aquatic insects can also be expected vary considerably. Again, despite a high degree of variation among and even within species, some generalizations are possible. For example, some fish species move only short distances in their natal stream or river during their lifetime, whereas other species exhibit large-scale migrations, such as in the case of anadromous salmon and trout species or in catadromous eel species (Matthews 1998). Some microorganisms are assumed to passively disperse large spatial distances, which has been suggested to result from the small size of their propagules easily moved by wind and other potential mediators of dispersal, as well as huge local population sizes facilitating high rates of emigration (Fenchel and Finlay 2004). However, any estimates of dispersal distances and rates of microorganisms should be considered with caution. Given the variation within well-known biological groups, such as among fishes only, one can only ponder how much variation there is in the dispersal distances and rates among species in microorganisms. It is well understood that there is a wide variation in body size among microorganisms, ranging from the smallest bacteria to the largest diatoms. Therefore, if one assumes that body size is associated with the dispersal capacity of an organism, it is necessary to consider the variation in dispersal-related traits among species within groups of microorganisms.
Most studies of riverine metacommunities suggest that dispersal, often indirectly measured by the spatial fraction in community compositional variation, is less influential compared with the sets of environmental factors considered by researchers (Heino and others 2015a). In a meta-analysis of several dozens of stream insect datasets, this was indeed found to be the case, even though a major portion of variation in community composition remained unexplained by the environmental variables used in the analyses (Heino and others 2015b). Yet, there were some discernible differences among the three stream insect groups considered, mayflies, stoneflies and caddisflies, as well as much context dependence resulting from different geographical settings, environmental conditions and environmental variables originally measured in a study area. Additionally, even across the same set of surveyed sites (i.e., keeping the spatial extent, geographical setting and environmental conditions as constant as possible), groups of organisms with presumably distinct dispersal abilities may show different degrees of spatial structuring and environmental control of community compositional variation.
Given that dispersal and connectivity are keys to understand metapopulation dynamics and metacommunity organization in riverine landscapes (Erős and Campbell Grant 2015), future studies should pay increasing attention to developing direct measures of dispersal. This could include development of cost-effective methods of mark-recapture of individual organisms using ‘quantum dot technologies’ (for a review of this technology, see Cotta 2020), or direct observations of the flight and movement of the organisms under study. Similarly, quantum dot technologies could also be applied in examining the fate and movement of matter in riverine metaecosystems. Although difficult in practice, advances in riverine metasystems ecology can more likely be gained by using such direct approaches compared with those based on proxies only (Heino and others 2017). In addition, field studies that sample the same set of sites continuously over time should be preferred over those that use the traditional ‘low-hanging fruit approach’, whereby the sites are sampled only once and various means of data analysis are used to indirectly infer the roles of dispersal and environmental factors for compositional variation in biotic communities in rivers and streams.
Key references
Allan, J.D., Castillo, M.M. & Capps, C.A. (2021). Stream Ecology. The Structure and Function of Running Waters. Springer, New York.
Altermatt, F. (2013). Diversity in riverine metacommunities: a network. perspective. Aquatic Ecology 47: 365–377.
Ament, R., Callahan, R., McClure, M. Reuling, M. & Tabor, G. (2014). Wildlife Connectivity: Fundamentals for Conservation Action. Center for Large Landscape Conservation. Bozeman, Montana.
Auerbach, D.A. & Poff, N.L. (2011). Spatiotemporal controls of simulated metacommunity dynamics in dendritic networks. Journal of the North American Benthological Society 30: 235-251.
Bellard, C. & Hugueny, B. (2020). Importance of metapopulation dynamics to explain fish persistence in a river system. Freshwater Biology: 65 1858– 1869.
Bilton D.T., Freeland J.R. & Okamura B. (2001). Dispersal in freshwater invertebrates. Annual Review of Ecology and Systematics: 32 159-181.
Brown, B.L. and Swan, C.M. (2010). Dendritic network structure constrains metacommunity properties in riverine ecosystems. Journal of Animal Ecology 79: 571-580.
Clarke, A., Mac Nally, R., Bond, N. & Lake, P.S. (2008). Macroinvertebrate diversity in headwater streams: a review. Freshwater Biology 53: 1707-1721.
Cotta, M.A. (2020) Quantum dots and their applications: What lies ahead? ACS Applied Nano Materials. 3: 4920-4924.
Cummins, K.W. & Klug, M.J. (1979). Feeding ecology of stream invertebrates. Annual Review of Ecology, Evolution, and Systematics 10: 147-172.
Erős, T. & Campbell Grant, E. H. (2015). Unifying research on the fragmentation of terrestrial and aquatic habitats: patches, connectivity and the matrix in riverscapes. Freshwater Biology 60: 1487-1501.
Fenchel, T. & Finlay, B.J. (2004). The ubiquity of small species: Patterns of local and global diversity. BioScience 54: 777–784.
Gotelli, N.J. & Taylor, C.M. (1999). Testing metapopulation models with stream-fish assemblages. Evolutionary Ecology Research 1: 835-845.
Heino, J., Grönroos, M., Soininen, J., Virtanen, R. & Muotka, T. (2012). Context dependency and metacommunity structuring in boreal headwater streams. Oikos 121: 537-544.
Heino, J., Melo, A.S., Siqueira, T., Soininen, J., Valanko, S. & Bini, L.M. (2015a). Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects. Freshwater Biology 60: 845-869.
Heino, J., Melo, A.S., Bini, L.M., Altermatt, F., Al-Shami, S.A, Angeler, D., Bonada, N., Brand, C., Callisto, M., Cottenie, K., Dangles, O., Dudgeon, D., Encalada, A., Göthe, E., Grönroos, M., Hamada, N., Jacobsen, D., Landeiro, V.L., Ligeiro, R., Martins, R.T., Miserendino, M. L., Md Rawi, C.S. Rodrigues, M., Roque, F.O., Sandin, L., Schmera, D., Sgarbi, L.F., Simaika, J., Siqueira, T., Thompson, R.M. & Townsend, C.R. (2015b). A comparative analysis reveals weak relationships between ecological factors and beta diversity of stream insect metacommunities at two spatial levels. Ecology and Evolution 5: 1235-1248.
Heino, J., Alahuhta, J., Ala-Hulkko, T., Antikainen, H., Bini, L.M., Bonada, N., Datry, T., Erős, T., Hjort, J., Kotavaara, O., Melo, A.S. & Soininen, J. (2017). Integrating dispersal proxies in ecological and environmental research in the freshwater realm. Environmental Reviews 25: 334-349.
Kärnä, O.-M., Grönroos, M., Antikainen, H., Hjort, J., Ilmonen, J., Paasivirta, L. & Heino, J. (2015). Inferring the effects of potential dispersal routes on the metacommunity structure of stream insects: as the crow flies, as the fish swims or as the fox runs? Journal of Animal Ecology 84: 1342-1353.
Li, Z., Xing, Y., Liu, Z., Chen, X., Jiang, X., Xie, Z. & Heino, J. (2020) Seasonal changes in metacommunity assembly mechanisms of benthic macroinvertebrates in a subtropical river basin. Science of the Total Environment 729: 139046.
Matthews, W.J. (1998) Patterns in Freshwater Fish Ecology. Chapman and Hall, New York.
Siqueira, T., Saito, V.S., Bini, L.M., Melo, A.S., Petsch, D.K., Landeiro, V.L., Tolonen, K.T., Jyrkänkallio-Mikkola, J., Soininen, J. & Heino, J. (2020). Community size can affect the signals of ecological drift and niche selection on biodiversity. Ecology 101: e03014.
Snåre, H., García-Girón, J., Alahuhta, J., Bini, L.M., Boda, P., Bonada, N., Brasil, L.S., Callisto, M., Castro, D.M.P., Chen, K., Csabai, Z., Datry, T., Domisch, S., García-Marquez, J.R., Floury, M., Friberg, N., Gill, B.A., González-Trujillo, J.D., Göthe, E., Haase, P., Hamada, N., Hill, M.J., Hjort, J., Juen, L., Jupke, J.F., Justino de Faria, A.P., Li, Z., Ligeiro, R., Linares, M.S., Andrade, A.L., Macedo, D.R., Mathers, K.L., Mellado-Diaz; A., Milosevic, D., Moya, N., Poff, N.L., Rolls, R.J., Roque, F.O., Saito, V.S., Sandin, L., Schäfer, R.B., Scotti, A., Siqueira, T., Martins, R.T., Valente-Neto, F., Wang, B., Wang, J., Xie, Z. & Heino, J. (2024). The relationships between biotic uniqueness and abiotic uniqueness are context dependent across drainage basins worldwide. Landscape Ecology 39: 84.
Soininen, J., Bartels, P., Heino, J., Luoto, M. & Hillebrand, H. (2015). Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience 65: 174-182.
Tonkin, J.D., Altermatt, F., Finn, D., Heino, J., Olden, J.D., Pauls, S.U. & Lytle, D.A. (2018). The role of dispersal in river network metacommunities: patterns, processes, and pathways. Freshwater Biology 63: 141-163.
Vannote, R.L., Minshall, W.G., Cummins, K.W., Sedell, J.R. & Cushing, C.E. (1980). The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130-137.
Wallace, J.B. & Webster, J.R. (1996). The role of macroinvertebrates in stream ecosystem function. Annual Review of Entomology 41: 115-139.
Ward, J. V. (1998). The four-dimensional nature of lotic ecosystems. Journal of the North American Benthological Society 8: 2-8.